What makes us different
At NeoGenomics, we understand the needs of pharmaceutical and biotechnology companies, and we align with our partners to support development of therapies that will save lives. With over a decade of experience, we are a trusted partner in oncology research and development, delivering solutions to advance your drug development and clinical trial programs for targeted therapies and emerging treatment modalities.
No matter the scale or business need, we deliver:
- Rapid project initiation with flexible, scalable support for evolving study needs
- Immediate access to experienced MDs, PhDs, and a dedicated project manager, ensuring project integrity
- Available regulatory and quality experts to support our pharma and biotech partners
- Certified pathology staff capable of performing US Food and Drug Administration (FDA)-approved companion diagnostic confirmations
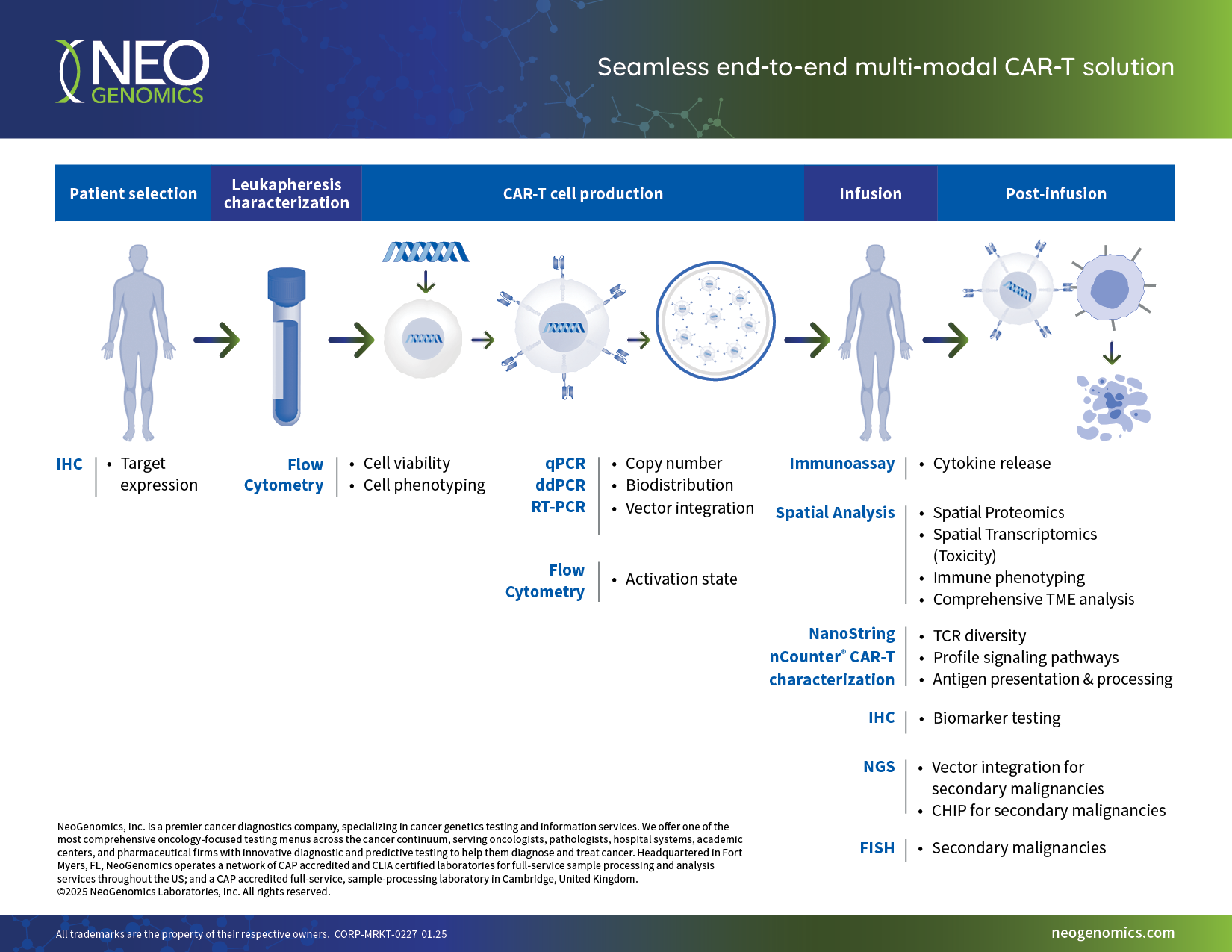
- Seamless end-to-end multi-modal solutions, eliminating the need for multiple vendors
- Broad pharmaceutical test menu and integrated data analysis
- Customized solutions to accelerate your development program