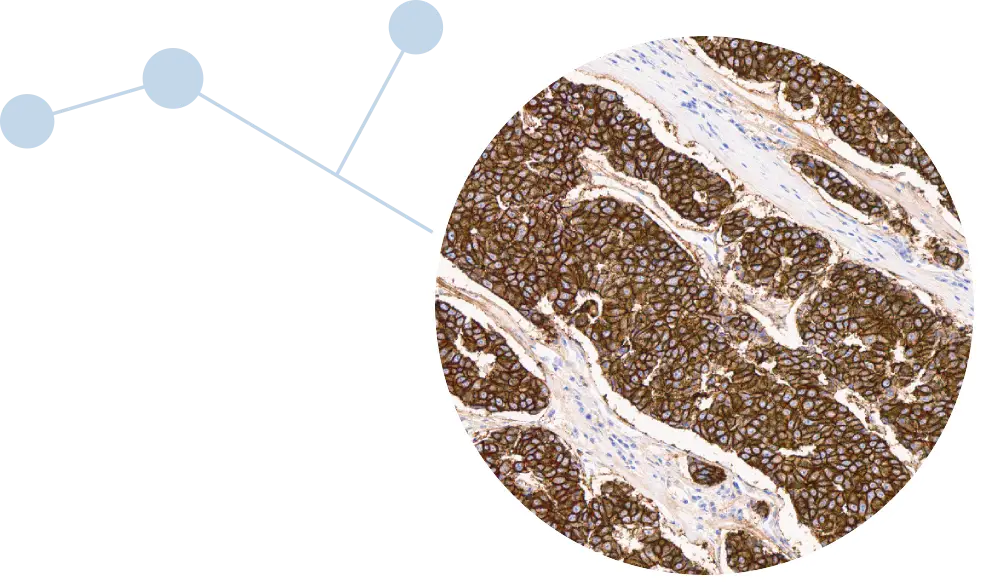
Accurate and timely biomarker testing is critical in lung cancer, where targeted therapies can meaningfully change the course of a patient’s treatment. The c-MET CDx for NSCLC assay detects c-Met protein overexpression, a biomarker observed in up to 50% of patients with advanced NSCLC.¹ It is designed to help identify patients who may be eligible for newly approved targeted therapies, including EMRELIS™ (telisotuzumab vedotin-tllv), which was recently approved by the U.S. Food and Drug Administration (FDA).*